A Single Genetic Tweak Stops Mosquitoes from Spreading Malaria
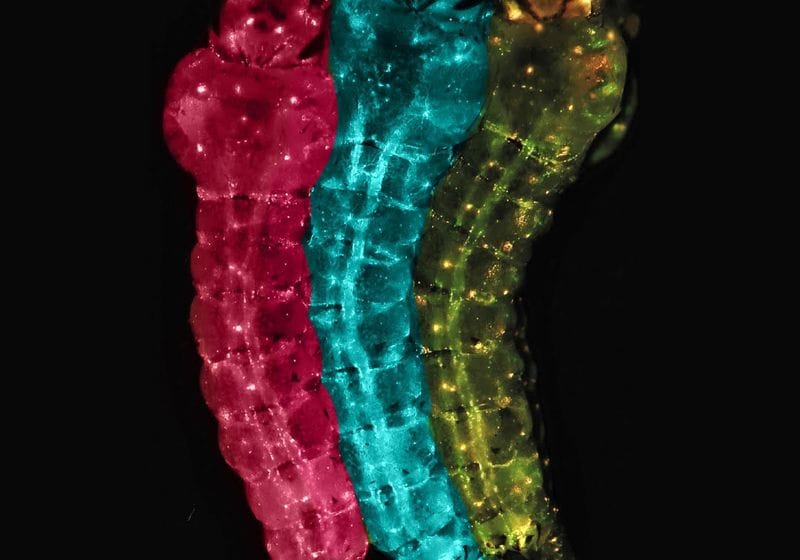
A new type of gene drive can render mosquitoes resistant to malaria infection. Mosquito larvae carry naturally occurring variants of the Refre1 gene that are either susceptible (pink or yellow) or refractory to parasite infection (blue).
Image credit:Zhiqian Li, Bier lab, UCSD
From insecticides to vaccines, scientists have developed a range of approaches to combat the spread of malaria, but they are yet to identify a broadly effective strategy. As mosquitoes adapt resistance to insecticides and the Plasmodium parasites they transmit become resistant to drugs, cases of malaria continue to creep higher, claiming hundreds of thousands of lives each year.
Now, a team of researchers from the University of California, San Diego (UCSD) and Johns Hopkins University has collaboratively devised a new strategy to stop the disease in its tracks. Using CRISPR-Cas9 gene editing technology, the researchers inserted a naturally occurring variant into a gene in mosquitos to render them immune to malaria infection. The results, published today in Naturedescribe a ‘phantom’ allelic drive in mosquitos that can disappear after it stops them from spreading the disease to humans.1
In previous studies, researchers have investigated the use of CRISPR-Cas9 gene editing technology to create gene drives in mosquitos. Gene drives are genetic elements introduced into the genome that spread rapidly through populations via biased inheritance. The goal is to reduce mosquito numbers or prevent them from being infected with the Plasmodium parasites.2
In the present study, developmental biologist Ethan Bier of UCSD and his colleagues took a slightly different approach to the concept of gene drives. “We (wanted) to change the structure of the existing (mosquito) population such that it carries a variant that’s naturally occurring, that is, one that renders the mosquitoes refractory to malaria transmission,” Bier explained.
To create malaria-resistant mosquitos, the team exploited a key protein that acts as a bottleneck in the process of Plasmodium infection. Fibrinogen-related protein 1, encoded by the Refre1 gene, has essential physiological functions in mosquitoes and is necessary for their survival. It also plays a crucial role in allowing the parasites to travel across the mosquito gut epithelium and into their salivary glands, from where they can then be transmitted to humans.3 A previous genome-wide association study had identified a naturally-occurring variant of Refre1 that makes Anopheles stephensi mosquitoes unsuitable vectors for Plasmodium.4
So, Bier and his team used CRISPR-Cas9 to create a population of mosquitos carrying this variant. “We generated isogenic strains of mosquitos, one of which had the permissive variant of the Refre1 gene that’s the so-called L224 allele, which is the most common one, and then there’s a naturally occurring Q224 variant,” said Bier.
That small difference—a single amino acid change from leucine to glutamine at the position 244 in the resulting protein—had a profound effect on the mosquitos: When the researchers tried to infect the mosquitos with Plasmodium falciparumthe highly lethal strain that dominates malaria infections in Africa, they observed that the mosquitos carrying the Refre1Q224 allele were almost completely resistant to infection. The variant blocked the parasites from traveling to the midgut without affecting the mosquitos’ overall fitness. But it also had another, somewhat unexpected benefit in further experiments: It also blocked infection from Plasmodium vivax, which is predominantly found in Southeast Asia but is only distantly related to A. falciparum.
Next, the team used CRISPR-Cas9 to create a system similar to a gene drive that would bias the inheritance of the Refre1Q224 allele in larger populations of mosquitos, conferring widespread resistance to Plasmodium parasites. The team had previously used this approach to ‘re-wild’ fruit flies, reversing their resistance to pesticides.5
While a traditional gene drive typically involves the insertion of an entire gene and will persist long-term in the population, the team’s ‘phantom’ allelic drive only changes a single allele of a gene and can be kept or eliminated from the population depending on long-term requirements. Bier said this approach circumvents the risks associated with gene drive technologies, such as the long-term persistence of these drives in wild populations and the chances of mutations arising. “You can insert the cassette in a gene where it incurs a fitness cost, and when you do that, over time it disappears,” he said. This means that the cassette won’t continue to spread, “but during the period of time when it’s present, it can exert its effect and surprisingly effectively.”
Bier remarked that this new approach is not a stand-alone measure but will be most successful when applied alongside other malaria prevention strategies, including mosquito population suppression. “The idea is to use them in combination with what’s out there in terms of the standard vector control mechanisms and have that whole package hopefully work together in a collaborative, cooperative way to solve this terrible problem,” Bier said.
Maciej Maselkowho studies genetic engineering in mosquitos at Macquarie University and was not involved in the study, was enthusiastic about the results. “These findings provide a promising path toward modifying wild mosquito populations to help in the battle against malaria,” Maselko said in an email. He added that he hopes to see this technology implemented in field trials in the near future. “Such potentially cost-effective interventions are urgently needed, especially at a time when funding for global public health is waning.”