Low Brain Lithium Leads to Alzheimer’s Disease Pathology
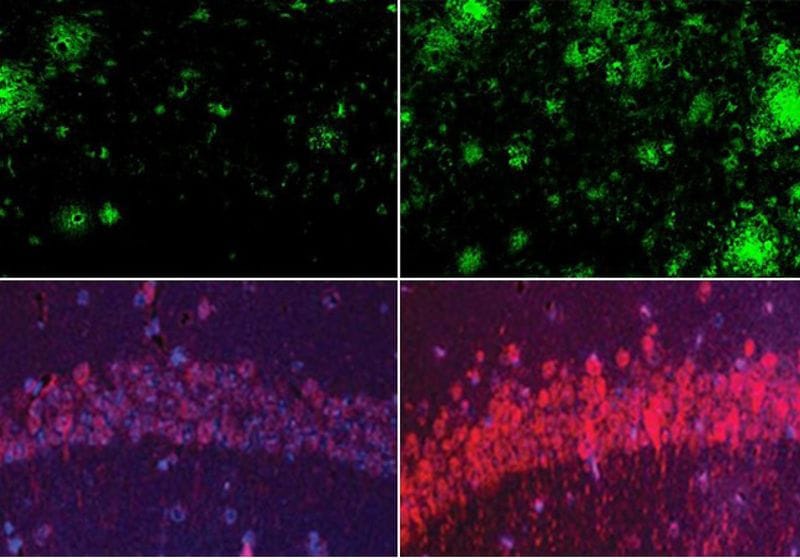
Lithium deficiency (right) increased Aβ deposits and tau protein levels in the mouse brain than mice with normal physiological levels of lithium (left).
Image credit:Yankner Lab
In Alzheimer’s disease, researchers have identified genetic variants and other risk factors related to diet, lifestyle, the environment, but metal levels in the brain also contribute to the condition.1 Imbalanced levels of iron, copper, and zinc can promote features of Alzheimer’s pathology such as amyloid-β (Aβ) aggregation, tau phosphorylation, and oxidative stress.2 However, there are other metals that contribute to brain function, and how these are affected or disrupted in Alzheimer’s disease is still largely unexplored.
To tackle this metal mystery further, researchers from Harvard Medical School and Rush University Medical Center teamed up. When they screened levels of metals in human brains and blood, one metal stood out. In their new study, published in Naturethe team found that lithium (Li) deficiency resulted in early physiological changes leading to Alzheimer’s disease.3 Meanwhile, Li supplementation in mice could suppress this pathology and restore memory, suggesting a potential approach to prevent and combat the disease.
“The idea that lithium deficiency could be a cause of Alzheimer’s disease is new and suggests a different therapeutic approach,” said study author Bruce Yanknera neurologist at Harvard Medical School, in a press release.
While Li levels were significantly reduced in individuals with mild cognitive impairment and Alzheimer’s disease, the researchers wanted to know if this was reflected in Aβ plaques. In human brain samples, the team found lower Li levels correlated with reduced cognitive function. Meanwhile, in a mouse model for Alzheimer’s disease, they found that Li accumulated and bound to Aβ plaques, reducing Li uptake in the brain.
To further study this deficiency, they compared wild type mice with two mouse models of varying Alzheimer’s pathology: one accumulates Aβ deposits and phosphorylated tau (phospho-tau), while the other accumulates Aβ deposits. When fed a Li-deficient diet for five weeks, healthy mice had lower Li levels, similar to those of patients with Alzheimer’s disease. The other groups developed accelerated pathology, as evidenced by increased Aβ, phospho-tau cells, the activation of inflammatory microglial cells, and cognitive decline.
Then, the researchers aimed to characterize the transcriptome effects in their Alzheimer’s disease mouse models fed either a lithium-deficient or a control diet. Using single-nucleus RNA sequencing, they found that Li levels altered the activity of genes known to raise or lower the risk of Alzheimer’s disease, including the most well-known risk factor, apolipoprotein E (Apoe).
Because Li appeared to have such an interconnected role in disease pathology, the researchers wondered if they could reverse its effects with Li replacement therapy. Another compound, lithium carbonate, has shown efficacy for Alzheimer’s disease in pre- and clinical studies, but it can be toxic at the high doses normally used.4 They used a screening platform to find a compound that might bypass Aβ so that it can avoid being sequestered and be an effective treatment. They chose lithium orotate (LiO) based on this criterion, and they gave it to mice at a low dose in their drinking water. “One of the most galvanizing findings for us was that there were profound effects at this exquisitely low dose,” Yankner said.
The researchers observed lower LiO levels in Aβ plaques and higher levels of Li in non-plaque areas of the brain. They also saw that this LiO brain distribution benefited older mice with advanced stages of the disease. LiO-treated mice had downregulated genes related to neurodegeneration and upregulated genes related to learning and memory.
“What impresses me the most about lithium is the widespread effect it has on the various manifestations of Alzheimer’s disease. I really have not seen anything quite like it all my years of working on this disease,” said Yankner.
Based on these findings, the researchers have shown that Li plays an essential role in normal brain function and can confer resistance to brain aging and Alzheimer’s disease in mice. Yankner acknowledged that further studies are needed but also expressed cautious optimism that studying Li levels may help scientists identify a clinical target level for patients receiving Li-based treatments in clinical trials.