FDA Announces Plan to Phase Out Animal Testing. Will That Work? New
Estimated reading time: 8 minutes
With the FDA’s goal of replacing animal testing, scientists look to organoids and organ-on-a-chip technologies as replacement alternatives.
Image credit:Wyss Institute at Harvard University
In April of this year, the Food and Drug Administration (FDA) announced a new roadmap that aims to replace animal testing in the development of new drugs with more human-relevant methods. The goal is to improve drug safety, accelerate the evaluation process, shorten drug development timelines, reduce costs, and spare animal lives. The FDA aims to make animal testing the exception rather than the norm within three to five years. Is this possible?
The FDA has required animal testing of new drugs since the 1930s after more than 125 American adults and children died after ingesting an antibiotic elixir that mistakenly contained the poison found in antifreeze—diethylene glycol—which was then thought to be just a sweetening agent. Animal testing remains the mainstay of drug evaluations by the FDA, and it undoubtedly has helped prevent other potentially dangerous chemicals from reaching patients. However, on the flip side, the results of drug tests in animals fail to predict future responses in humans more than 90 percent of the time.1 It is also likely that many drugs that could have been safe and effective in humans never received approval because they were found to be toxic in early animal studies. Aspirin is a great example; we are all lucky because it was first marketed before 1900.2
The scientific and ethical limitations of animal testing have received progressively more attention over the past twenty years. Finally, in December 2022, Congress passed the FDA Modernization Act 2.0 which authorized the agency to use data generated with advanced human cell-based assays and computer models as alternatives to animal testing when considering the safety and effectiveness of a drug. But since that time, Congress has seen little evidence that the FDA is walking the walk along this path. As a result, it has been considering passage of the FDA Modernization Act 3.0 that compels the agency to take more active steps to phase out animal studies. This is precisely what the new administration chose to do on its own in April.
Artificial Intelligence Could Help Replace Animal Models…But Not Right Away
While the FDA’s new accelerated timeline for replacing animal testing is aspirational, they believe that recent advances in artificial intelligence (AI)-based computational models and in new advanced human cell culture models, namely organoid and organ-on-a-chip (organ chip) technologies could now make this a possibility.3,4 While AI could potentially dive through heaps of data to pull out drug candidates that are safe and effective in humans in the future, the field is still in its infancy. More importantly, AI models are only as good as the data they ingest, and predictions made based purely on existing data from the web and past publications are essentially computer-based correlations or “guilt-by-association.” The true power of this approach will be tested when AI predictions can be tested in human-relevant experimental assays, with the data being fed back into the model to refine and optimize predictions through iteration. At present, drugs being developed with AI models still require validation through testing in animals, and now potentially in human organoid or organ chip models.
Human Organoids and Organ Chips as Alternatives to Animal Testing
The FDA’s proposal to explore human organoid and organ chip technologies, along with AI, is timely and wise because these different approaches can synergize to produce results that are highly predictive of human drug responses. Organoids are small balls of stem cells isolated from human patients that reform specialized tissue structures and functions, such as finger-like intestinal villi, when grown within a Jello-like matrix and bathed in a nutrient medium. Because these organoid cultures express the molecular machinery of human cells rather than rat or dog cells, they are much more relevant testbeds for both mechanistic studies and for testing drugs that are designed to go into humans. But the same human cells that grow within these organoids behave differently when they are naturally nestled close to connective tissue containing blood vessels, contact immune cells, and experience physical forces associated with blood flow, breathing motions, and body movements as they do within living organs inside our bodies.
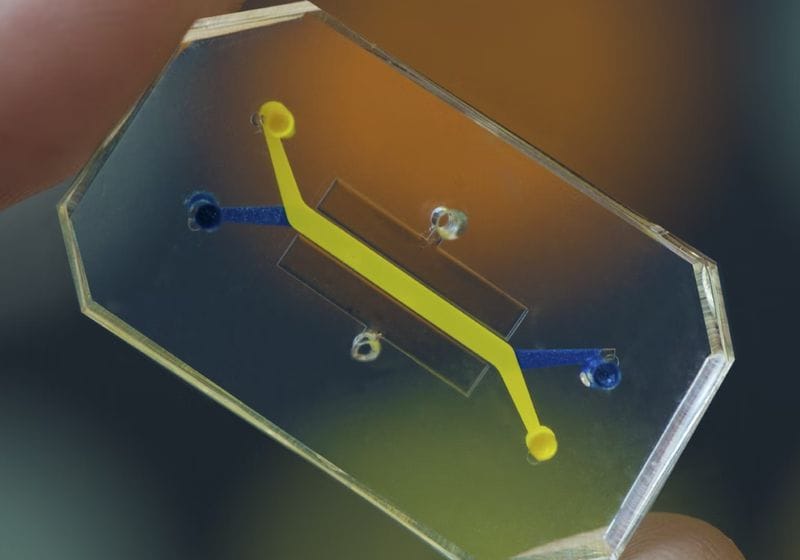
Researchers use organ chips to closely mimic tissue environments. They can test disease responses to further their understanding and develop therapies.
Wyss Institute at Harvard University
Organ chips provide a much more complex and in vivo-like culture environment for all types of human cells, including cells isolated from organoids. These chips are optically clear devices the size of a USB thumb drive made from a flexible rubber-like material that contain tiny hollow channels lined by living human cells from multiple tissues. The different types of tissue cells are positioned to recreate organ-level structures, dynamically perfused with a blood-like medium, and rhythmically deformed, for example, to mimic breathing in the lungs or peristalsis in the intestine. Because these chips experience fluid flow, drugs can be perfused through their vascular channels much like in our bodies, and drug levels can be varied over time to replicate changes that we experience when we take a drug once versus three times a day. Lung cells can be cultured in contact with air, intestinal cells can be grown with a living human gut microbiome, and skin cells can be stretched and relaxed as they do when we move our bodies. Fluid samples collected from both channels also can be probed with state-of-the-art analytical tools to identify molecules associated with inflammation or discover new disease biomarkers that might be found in blood, stool, urine, or vaginal fluid, depending on the type of cells and tissues that inhabit the chips. Organ chips lined with cells from patients with chronic diseases, such as inflammatory bowel disease or chronic obstructive pulmonary disease (COPD), as well as rare genetic disorders of childhood, faithfully mimic the symptoms that patients with these diseases experience. Lung chips infected with different strains of influenza virus recapitulate viral replication, lung injury, and inflammatory responses, and differences in virulence are detected, all of which are seen in human patients. Chips lined with cells from COPD patients who experience exacerbations of their disease when they contract a viral infection similarly exhibit a higher degree of viral replication and inflammation in the chip. Most importantly, when drugs are administered to these human organ chips using clinically relevant doses, frequencies, and routes of administration (intravenously through the vascular channel versus orally via the villi-lined channel of the intestine chip), the dose sensitivity, safety, and efficacy responses often closely replicate what is seen in human patients.
The ability to model human disease states in men versus women, elderly versus children, different ethnic groups, and distinct genetic subpopulations by creating organoids or lining organ chips with cells isolated from these patients is a game-changer. This is because preclinical animal testing that the FDA currently requires for drug safety assessments is usually carried out in healthy rats, dogs, or non-human primates. Indeed, a recent study cited in the FDA’s roadmap involved human liver chips treated with 27 different drugs whose effects in both past animal and human safety studies were known. The organ chips were found to be many times more accurate at predicting drug-induced liver injury in humans than the past animal experiments.5 This same study carried out an economic analysis and estimated that replacing this one type of animal test could save the pharmaceutical industry—and potentially patients—approximately two to three billion dollars per year by preventing failures in late-stage clinical trials. A scientist at Moderna also recently reported that they are using the same liver chip model to determine the safety of lipid nanoparticle delivery systems for RNA therapeutics instead of non-human primates. Because non-human primates are extremely expensive, they are able to carry out these organ chip safety studies at less than one-tenth the cost and in one-fourth the time.
The Future of Animal Testing Depends on FDA’s Follow-Through
Organoids and organ chips are beginning to be integrated into drug development pipelines to reduce animal usage and increase the likelihood of success. Are they going to completely replace animal testing in three to five years? This is unlikely as each new type of human culture model needs to be qualified or validated in terms of its ability to predict human responses within a specific context of use (drug-induced liver injury or monoclonal antibody-induced inflammatory reaction) with high fidelity, much like was done with the human liver chip. However, with this new move by the FDA, which provides incentives to pharmaceutical companies in the form of expedited reviews of their investigational new drug applications if they include data from these human-relevant experimental models, there is an excellent chance that we will see significant reductions in animal usage that will accelerate over time. This will save the lives of animals and enable the development of safer and more effective drugs faster and at a lower cost. Even the National Institutes of Health is recognizing the need to switch over to these types of alternative models that are more human-relevant, as they have just announced that they will no longer award funding to grant proposals that rely exclusively on animal testing. Now we just need to see that the FDA walks the walk.




